Roles of the egg jelly and vitelline layer
Despite the significant structural diversity of eggs and sperm of the animal species and their different physiological properties, one of the ultimate events of the fertilization process, which depends on the species-specific recognition, is the fusion of the sperm with the egg plasma membrane to transduce the fertilization signals. The sequence of the characteristic cellular and molecular events comprising the fertilization process has been intensively studied in sea urchin. The fertilizing sperm must undergo morphological modifications (the acrosome reaction, AR) upon reaching the outer gelatinous layer enveloping the egg (egg jelly), which triggers the polymerization of F-actin on the sperm head to form the acrosomal process. To investigate the specific roles of the egg jelly and vitelline layer at fertilization, sea urchin (Paracentrotus lividus) eggs were incubated in acidic seawater, which removes the egg jelly, i.e., experimental conditions that should prevent the occurrence of the AR, and inseminated in the same medium. At variance with the prevailing view, our results have shown that these dejellied P. lividus eggs can still interact with sperm in acidic seawater, albeit with altered fertilization responses.
Moreover, eggs deprived of the vitelline layer reacted to multiple sperm but with altered Ca2+ signals. The results have provided experimental evidence that the plasma membrane, not the vitelline layer, is where the specific recognition between gametes occurs. The results also indicated that the vitelline layer serves to prevent polyspermy, as its removal promotes the entry of multiple sperm. Thus, the structural integrity of the vitelline layer covering the unfertilized eggs is essential to mask the fusogenic sites residing in the plasma membrane, the exposure of which would induce homologous polyspermic fertilization. Our results on the requirement of specific receptors on the plasma membrane and not the vitelline layer for the specific recognition and fusion with sperm raise the question about the exact role played by the vitelline layer in eggs of different species and the molecular mechanisms regulating the fertilization processes in other animal groups.
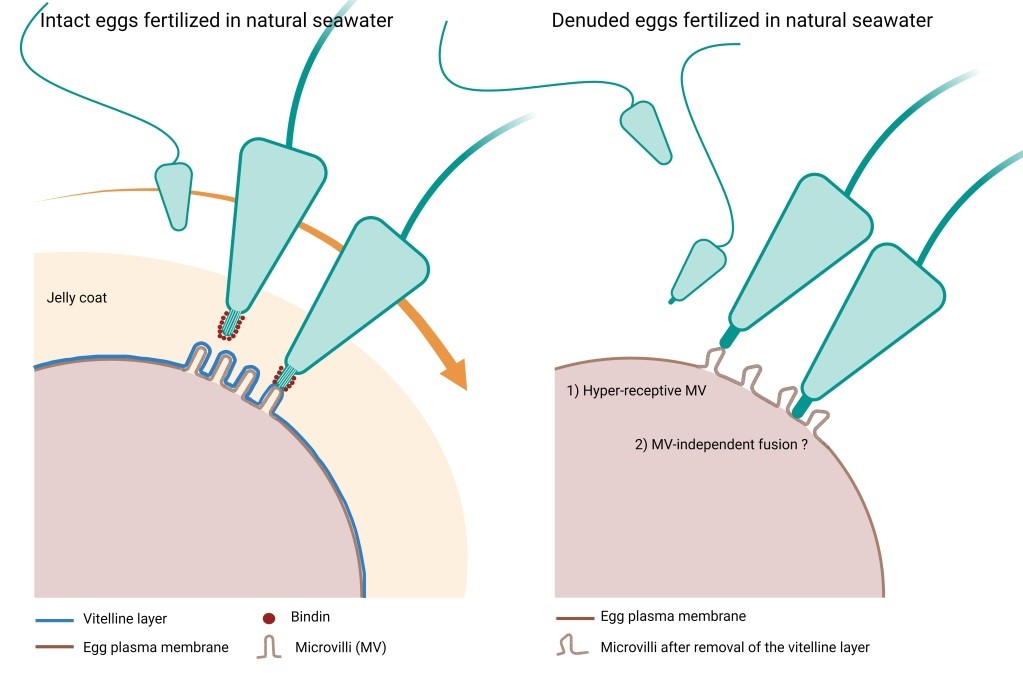 Fertilization in natural seawater of intact and denuded P. lividus eggs. Microvilli on the plasma membrane of intact sea urchin eggs are tightly covered by the vitelline layer that is surrounded by the jelly coat. According to the prevailing view, when the fertilizing sperm reaches the jelly coat, it undergoes morpho-functional changes owing to the polymerization of actin in the sperm head, which forms the acrosomal process. An adhesive protein ‘bindin’ is now exposed on the acrosomal filament and mediates the species-specific recognition and fusion with the vitelline layer of the egg. Our results have shown that removing the vitelline layer and egg jelly still promotes sperm fusion, leading to multiple sperm entries into the eggs of the same species despite the absence of the acrosomal filament formation due to the lack of the inducer jelly coat. By contrast, the denuded eggs fail to incorporate sperm of other sea urchin species. Thus, species-specificity control between sea urchin gametes resides at the plasma membrane level, not at the vitelline layer. The vitelline layer may serve to inhibit polyspermic fertilization.
Fertilization in natural seawater of intact and denuded P. lividus eggs. Microvilli on the plasma membrane of intact sea urchin eggs are tightly covered by the vitelline layer that is surrounded by the jelly coat. According to the prevailing view, when the fertilizing sperm reaches the jelly coat, it undergoes morpho-functional changes owing to the polymerization of actin in the sperm head, which forms the acrosomal process. An adhesive protein ‘bindin’ is now exposed on the acrosomal filament and mediates the species-specific recognition and fusion with the vitelline layer of the egg. Our results have shown that removing the vitelline layer and egg jelly still promotes sperm fusion, leading to multiple sperm entries into the eggs of the same species despite the absence of the acrosomal filament formation due to the lack of the inducer jelly coat. By contrast, the denuded eggs fail to incorporate sperm of other sea urchin species. Thus, species-specificity control between sea urchin gametes resides at the plasma membrane level, not at the vitelline layer. The vitelline layer may serve to inhibit polyspermic fertilization.
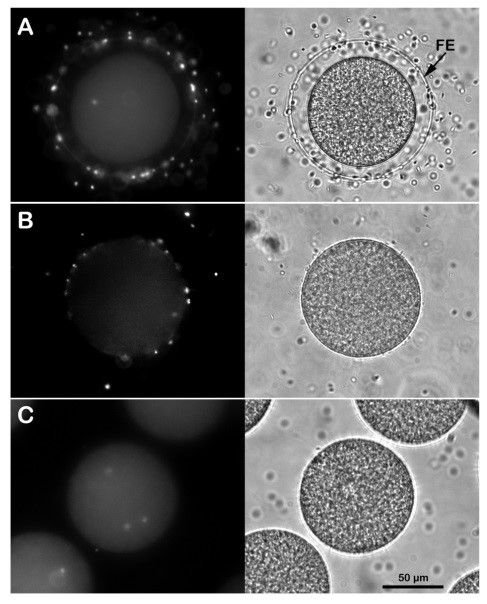
Effect of removing the egg jelly and vitelline layers on the sperm entry. (A) Intact P. lividus eggs inseminated in NSW with DNA-stained sperm are all penetrated by a single one, as shown by the UV laser in epifluorescence microscopy 5 minutes after insemination. The FE is fully elevated around the zygote, as shown in the transmitted light image. Monospermic fertilization or inhibition of sperm entry is observed in P. lividus eggs incubated for 5 minutes in acidic seawater (pH 5.5) and fertilized in the same medium (B). Note that at variance with the control in A, it is difficult to visualize the elevation of the FE in eggs fertilized in acidic seawater due to the structural modification of the egg surface induced by the lower pH of seawater. Multiple homologous sperm incorporation is observed when the P. lividus eggs are pre-treated with 10 mM DTT at pH 9 for 20 min to remove the egg’s layers and fertilized in NSW (C).
Limatola N, Chun JT, Santella L. Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer. September 2022Cells 11(19):2984.
Related References
Epel D Weaver, A M, Mazia D. Methods for removal of the vitelline membrane of sea urchin eggs. Exp. Cell Res. 1970, 61, 64-68. doi:10.1016/0014-4827(70)90257-0.
Lillie FR. The production of sperm-isoagglutinins by ova. Science 1912, 36, 527-530. doi: 10.1126/science.36.929.527.
Limatola N, Chun JT, Santella L. Regulation of the actin cytoskeleton-linked Ca2+ signaling by intracellular pH in fertilized eggs of sea urchin. Cells 2022, 11, 149. doi: 10.3390/cells11091496.
Limatola N, Chun JT, Cherraben S, Schmitt JL, Lehn JM, Santella L. Effects of Dithiothreitol on fertilization and early development in sea urchin. Cells. 2021, 10, 3573. doi: 10.3390/cells10123573.
Tilney LG, Hatano S, Ishikawa H, Mooseker MS. The polymerization of actin: its role in the generation of the acrosomal process of certain echinoderm sperm. J. Cell Biol. 1973, 59, 109-126. doi: 10.1083/jcb.59.1.109.
Vacquier VD, Moy GW. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 1977, 74, 2456-2460. doi: 10.1073/pnas.74.6.2456.
Vacquier VD, Tegner MJ, Epel D. Protease activity establishes the block against polyspermy in sea urchin eggs. Nature 1972, 240, 352-353. doi: 10.1038/240352a0.
Wessel GM, Wada Y, Yajima M, KiyomotoM. Bindin is essential for fertilization in the sea urchin. Proc. Natl. Acad. Sci. USA . 2021, 24;118:e2109636118.