Responsible: Raffaella Casotti
Activities
The laboratory of Microbial Ecology carries on research on marine microorganisms (mainly diatoms and autotrophic/heterotrophic picoplankton) studying their diversity, activity and metabolic functions as well as their interactions with the environment, both physical-chemical and biological. The activities are held mainly by direct methods, that is, independent from cultivation, but monospecific cultures are also used to test specific hypotheses or further characterize microorganisms. The fields of activity cover the biological oceanography, ecophysiology and marine ecology in general.
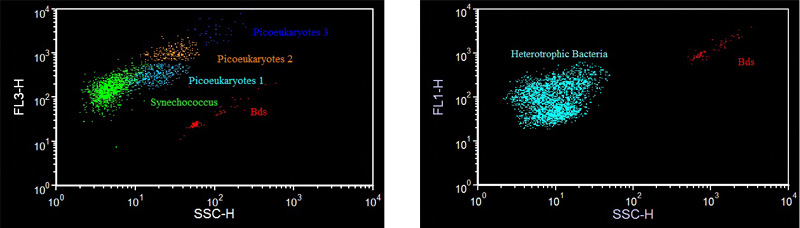
The laboratory also implements the technical and instrumental approaches for the study of microorganisms, mainly as regards the use of flow cytometry for the monitoring of marine plankton and for the development of early warning systems for biological environmental risk.
Experimental systems
- Thermostated cabinets for growing microalgae and bacteria under controlled light and temperature conditions.
- Tanks for experiments on board ships or outdoors for incubations and manipulations of natural plankton communities.
Analysis
- Counts and measurements of optical parameters (scatter and fluorescence) on cells and unicellular organisms by flow cytometry and epifluorescence microscopy.
- Estimates of growth rates and grazing of picoplankton.
- In situ hybridization with oligonucleotide probes for the characterization of bacterial communities.
- Estimates of DNA content of algae and cell lines by flow cytometry (including dynamics of the cell cycle).
- DNA and RNA extraction for metagenomics and metatranscriptomics.
- Analysis of cellular concentrations of picophytoplankton with scanning flow cytometers at high frequency in both laboratory and on board boats.
- Analysis of concentrations and cellular properties of marine bacteria with prototype automatic coloring module.
Instrumentation
- Conventional flow cytometry.
- Scanning flow cytometer with automated sampling module.
- Laminar flow hood.
- Thermostated cabinets for algal and bacterial cultures.
- Epifluorescence microscope with camera.
- Basic instrumentation for laboratory (centrifuges, thermostated baths, pHmeter, filtration rigs, sonicators, refrigerators and freezers).



Coordinator: Gabriele Procaccini
Scientific activities
Strumentazione di base per la biologia molecolare (PCR, centrifughe, apparati elettroforetici, transilluminatore, bagni termostatici, spettrofotometro, fluorimetro, pH-metro, bilancia di precisione)
Gene gun (apparecchio per la trasformazione biolistica)
In the laboratory of Molecular Ecology and Functional Genomics we carry out studies addressing ecological questions, using molecular approaches. The laboratory covers research activities ranging from molecular phylogeny and taxonomy to phylogeography and population genetics, using sequence analysis and analysis of polymorphic markers, such as microsatellites and SNPs. It also deals with the study of gene function through functional genomics approaches and reverse genetics (mainly production of transgenic lines of diatoms). We also makes use of analysis of environmental genomics and comparative transcriptomics, and we carry out gene expression studies in natural and controlled conditions (RNA-Seq, RT-qPCR).
Experimental systems
- Mesocosms for maintaining benthic organisms under controlled conditions.
- Walk-in chamber for microalgal cultures.
Analysis
- DNA extraction, purification and quantification.
- RNA extraction, purification, quantification and reverse transcription.
- Protein extraction and quantification.
- Sample preparation for RT-qPCR in plates.
- Samples preparation for massive sequencing.
- Samples preparation for sequence analysis.
- Samples preparation for fragments analysis.
- single and multiplex PCR.
- Electrophoresis on agarose and acrylamide gel.
- Statistical analysis of population genetics and molecular phylogeny.
- Primers design, analysis of electropherograms.
- Cloning and bacterial transformation.
- Preparation of plasmid DNA (mini and maxi prep).
- Biolistic transformation and cultivation of GM crops.
- Algal cultures.
- Colorimetric assays with fluorescent vital dyes and microscopic analysis.
- Southern blot, Northern blot.
- Western blot and enzyme assays (colorimetric, ELISA).
- Use of specialized software for the analysis of protein and nucleotide databases and for the exploration of genomes.
Equipments
- Basic equipment for molecular biology (PCR, centrifuges, electrophoresis apparatus, transilluminator, thermostatic baths, spectrophotometer, fluorometer, pH meter, balance).
- Gene gun (apparatus for biolistic transformation).
- Hoods for algal cultures.
- Microscopes.
- Mixer Mill (Tissue Lyser).
- PC and software for molecular analysis.
- Fume hood.
- TurboGen (equipment for generating marine turbulence).
Head: Marina Montresor
Activities
The lab contains equipment and infrastructure used for the study of the functional and taxonomic diversity of marine plankton. In particular, in this laboratory analyses are carried out on the taxonomic and molecular diversity of plankton samples collected at the Long Term Ecological Research site MareChiara in the Gulf of Naples as well as elsewhere, in the frame of participation in regional and global projects. Experimental approaches focus on the physiology and life cycles of marine microalgae and on the feeding behaviour of zooplankton.
Systems for experimentation
- Light- and temperature-controlled walk-in incubator and incubators of different volume for in vivo experiments with phyto- and zooplankton.
- Optical system for video footage of zooplankton organisms.
- Plankton wheel for experimentation with phyto-, micro- and mesozooplankton.
Analysis
- Quantitative analysis of marine phytoplankton and zooplankton.
- Preparation of phytoplankton cultures and their morphological and molecular characterization.
- Phylogenetic analysis.
- "Metabarcode" analysis of marine plankton with Next Generation Sequencing approaches.
Instrumentation
- Stereoscopes and optical microscopes
- Microscope with epifluorescence illumination and digital cameras to take photographs and movies.
- Zooscan with related software.
- Camera System for video-registration of zooplankton movement.
- Area equipped for the isolation of cultures and experimentation (laminar flow hood, microscopes for cell isolation, fluorimeter).
- Molecular Laboratory equipped for DNA extraction, PCR, DNA electrophoresis, preparation for NGS sequencing.









Coordinator: Daniele Iudicone
Activities
The aim of the laboratory is to support the study of the ecology of marine plankton and of the ocean physics that is relevant for the plankton ecology. The laboratory of ecological modeling has as main activity the preparation and realization of numerical simulations of the ocean circulation at microscale (DNS), mesoscale (ROMS) and at global scale (NEMO) and of the associated plankton dynamics. The activities are integrated with statistical analysis of environmental (physicochemical) data, ecological (populations) data and biological (metagenomics, functional parameters) data.
Experimental systems
- In-house configurations of numerical codes for simulating the ocean circulation, the fluid dynamics at the microscale (turbulence) and the impact on microorganisms.
- In-house configurations of numerical codes for simulating planktonic populations at different levels of complexity.
- Turbulence Profiler.
- Devices for the production of marine turbulence in lab (TurboGen).
Analysis
- Analysis of the variability of the ocean physics (including thermodynamics) and biochemistry at various spatial and temporal scales at basin and sub-basin scales.
- Statistical analysis of environmental data and ecological.
- Water mass thermodynamic budgets.
Instrumentation
- Servers and computers for numerical simulations.
- Software for data analysis.
- Software for the simulation of the physics and biology of marine organisms (produced in-house or by external collaborators).


Coordinator: Francesco Paolo Patti
Activities
In the Laboratory of Integrative Taxonomy of Marine Organisms the activities are based on the analysis of classic taxonomy, phylogeny and comparative phylogeography of marine organisms by means of a multidisciplinary integrated approach (morphological, molecular, cellular and ecological). Following the main activities:
- Sample sorting, identification and counting in ecological and monitoring projects (e.g. Marine Strategy).
- Expert identification of organisms using traditional and advanced morphological and molecular methods (including preparation and deposition of genetic and ecological vouchers).
- Extraction and storage of DNA from different organisms (seaweeds and marine invertebrates tissue).
- RNA extraction from different organisms (seaweeds and marine invertebrates tissue).
- Extraction of secondary metabolites.
- Mini-gel electrophoresis and digital documentation.
- PCR amplification of DNA.
- Molecular cloning of target DNA segments (plasmid preps, restriction and PCR analysis).
- Real-Time fluorescent-based polymerase chain reaction.
- DNA barcoding.
- Terminal Restriction Fragment Length Polymorphism.
- DNA sequencing of fragments (in collaboration with SZN Molecular Biology and Bioinformatics Unit).
- Analysis of DNA sequences including the reconstruction of phylogenetic trees.
Systems for the experimentation
- Dedicated lab protocols.
- Bioinformatic systems analysis: 1) study of nucleotide variability (ad-hoc open source software for metadata analysis); 2) decomposing morphotypes forms into size and shape by geometric morphometric methods.
Analysis
- Analysis of DNA sequences including the reconstruction of phylogenetic trees.
- Analysis of the morphological variability and the genetic polymorphism.
- Analysis of allele frequency and genotype distribution.
- Analysis of terminal restriction Fragment Length Polymorphism.
- Preparation of samples for morphological analysis (using dedicated buffer solutions for microscope observations - Stereomicroscope and Scanning Electron Microscope) and molecular analysis (cryopreservation, standards and RNAlater fixatives for tissue preservation).
Equipment
- Stereomicroscope.
- Vibratome.
- Rotavapor.
- Chemical fume hood.
- Laminar flow hood.
- Mini-Gel horizontal electrophoresis systems.
- Refrigerated Bench-top Centrifuge.
- Spectrophotometer "Genequant Pro Classic".
- PCRs (Techne- Euroclone).
- RTPCR BIO RAD Opticon 2.
- Incubator for cell cultures.
- Analytical balance.
- Temperature controlled water bath (5l, +30°C/+120°C).
- Bench-top vortex mixer with general purpose head.
- Vertical laboratory autoclave and steam sterilizer.
- Laboratory Ice Machine.
- Liquid nitrogen dewars (5l+30l).
- Vertical Ultrafreezer -86°C.
- Vertical Freezers -20°C.
- UVP BioDoc imaging workstations for gel documentation and fluorescent imaging.
- Dedicated computer for phylogenetic reconstruction and data mining.